Research & Development Center
Innovation & Technology Division
YANMAR HOLDINGS CO., LTD.
YANMAR Technical Review
Effects of Reducing GHG Emissions: Calculation and Experimental Study of Fundamental Characteristics of Ammonia-Diesel Dual-Fuel Engines
Abstract
While NH3 is a promising substitute for fossil fuels, its low burning speed and low ignitability are major challenges. One way to address this in internal combustion engines is to mix it with diesel fuel. This study uses both numerical simulation and experiments on a dual-fuel engine to investigate the effects of the mixing ratio and air-fuel ratio on combustion and emission characteristics. The results show that the air-fuel ratio influences emissions of NO+NO2, NH3, and N2O, but that it is difficult to prevent them completely through combustion improvements alone. This indicates that aftertreatment systems will be essential for NH3 internal combustion engines.
1.Introduction
Carbon-free fuels such as hydrogen and ammonia (NH3) are attracting attention due to the need to reduce greenhouse gas (GHG) emissions on a global level. Easier to store than hydrogen, and with a higher energy density, ammonia is being investigated for use in ocean-going vessels that need to travel long distances. Unfortunately, in addition to its high toxicity, the practical application of ammonia is complicated by its low burning speed and low ignitability, and by the fact that its combustion results in the emission of N2O (which has a high global warming coefficient) and NO (an atmospheric pollutant).
Yanmar has drawn on its expertise in combustion to identify improvement measures for overcoming these challenges. This article describes a research study done on practical applications of ammonia using simulation and experimental approaches.
2.Elucidation of Ammonia Combustion Products through Reaction Simulation
2.1.Numerical Simulation
A zero-dimensional chemical reaction simulation was undertaken to determine the combustion products of ammonia under the temperatures and pressures likely to be found in a compression ignition engine. Cantera was used as the reaction solver and Nakamura et al.'s model(1) was used for the ammonia reaction mechanism. The reaction was simulated under conditions of constant temperature and pressure, without considering how these values would change over the course of the reaction. A pressure of 6.0 MPa was used, indicative of the likely conditions inside the cylinder of a compression ignition engine when combustion is initiated. The calculation time was 1.0 ms and a φ-T map(2) was created to study their characteristics of the combustion products at the completion of the simulation.
2.2.Simulation Results
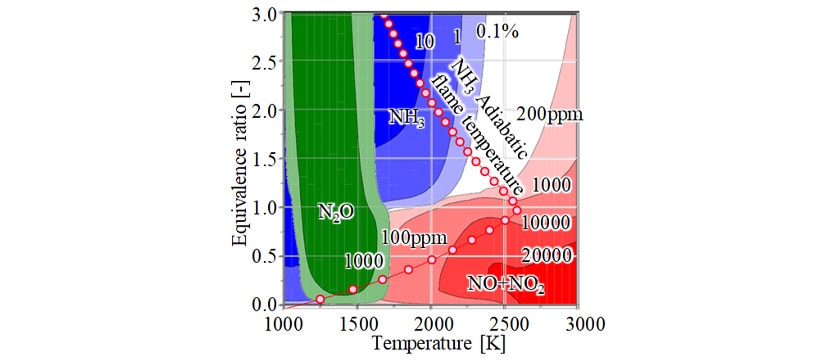
Fig. 1 shows a reaction product map of the quantities of NO+NO2, unburned NH3, and N2O in the ammonia-air mixture, and how the adiabatic flame temperature of the ammonia-air mixture varies with the equivalence ratio (for a pressure of 6 MPa and initial temperature of 1000 K). This indicates that NO+NO2 is produced across a wide range of conditions from low to high temperature. In the φ-T map(2) for a diesel-air mixture published by Akihama et al., NO is produced at high temperatures above 2000 K, which is assumed to be largely due to thermal NO. In the ammonia-air mixture, however, because NO is also produced in low-temperature conditions below 2000 K, fuel NOX (NOX that derives from the nitrogen in the fuel) plays a large part in the production of NO+NO2 at these lower temperatures. For equivalence ratios below 1.0, unburned NH3 occurs at low temperatures below 1500 K. At equivalence ratios above 1.0, it occurs at temperatures below 2200 K. N2O, meanwhile, tends to increase at temperatures around 1500 K, regardless of the equivalence ratio.
These results indicate that conventional lean combustion, running lean with an equivalence ratio of around 0.5, will not minimize NO+NO2, and that the lower combustion temperature will likely increase the amounts of unburned NH3 and N2O. The results also indicate the existence of a region where the quantities of NO+NO2, unburned NH3, and N2O are all kept low by running rich with an equivalence ratio in the 1.2-to-1.5 range. These results agree with those obtained from a numerical simulation of a one-dimensional planar flame by Kobayashi et al.(3). From this, it can be concluded that a high equivalence ratio and high-temperature combustion can simultaneously inhibit emissions of NO+NO2, unburned NH3, and N2O from ammonia combustion.
3.Elucidation of Basic Combustion Characteristics through Ammonia-Diesel Co-firing Experiment
3.1.Engine and Experimental Conditions
Fig. 2 shows a diagram of the experimental setup. Liquefied ammonia from a cylinder was vaporized and continuously supplied to the upstream end of the intake manifold while regulating the flow rate with a mass flow controller. The engine used in the experiment was a general-purpose single-cylinder diesel engine with a 94-mm bore and 110-mm stroke. A diesel-ammonia co-firing experiment was conducted by injecting diesel fuel directly into the cylinder using a common rail direct injection system. An external air compressor was used on the air intake to achieve the required temperature and enable the pressure to be adjusted independently of the other engine operating conditions. A Fourier-transform infrared (FT-IR) spectrometer was used to analyze the composition of the exhaust gas.
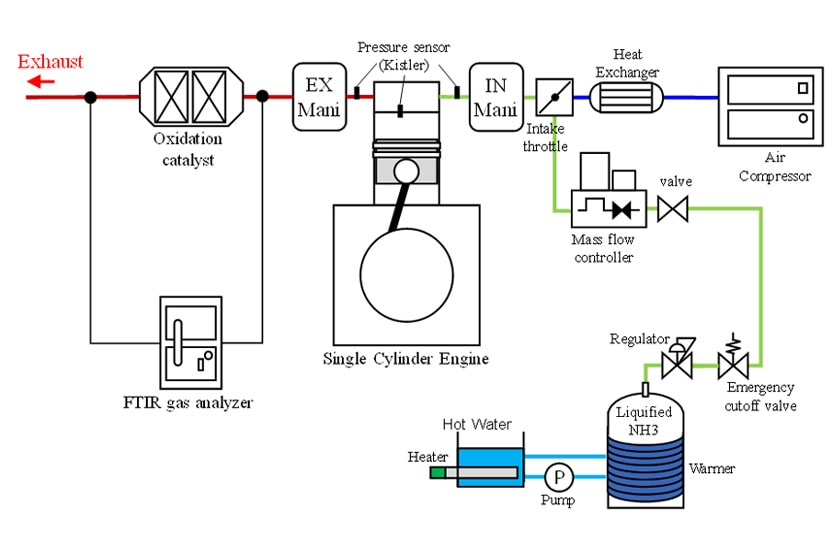
This study involved varying the amounts of ammonia and diesel while operating the engine at a constant speed of 1200 min-1 with a constant load at 1.0 MPa defined in terms of indicated mean effective pressure (IMEP). The ammonia-diesel mixing ratio was defined by equation (1), below. Here, 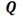 is the input calorific value, with subscripts
is the input calorific value, with subscripts  and
and 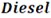 indicating the respective fuels.
indicating the respective fuels.
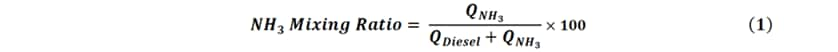
To vary the air-fuel ratio, the intake air amount was regulated by adjusting the pressure of the external air compressor.
3.2.Experimental Results and Discussion
3.2.1.Effects of NH3 Mixing Ratio
To study how the mixing ratio affects the exhaust of the ammonia-diesel mixed-fuel engine, testing was conducted using mixing ratios of up to 95 % ammonia. Fig. 3 shows the results for NH3 combustion efficiency, exhaust composition, and GHG emissions. NH3 combustion efficiency is defined by the following equation (2). Here, the 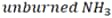 subscript refers to unburned NH3 in the exhaust.
subscript refers to unburned NH3 in the exhaust.
The values for GHG emissions are relative values compared to running on diesel only and are calculated using the 100-year global warming potential (GWP100) data published in the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC). In AR5, the GWP100 value for N2O is 265 times that of CO2. The figures for NO+NO2 are likewise relative values compared to running on diesel only.
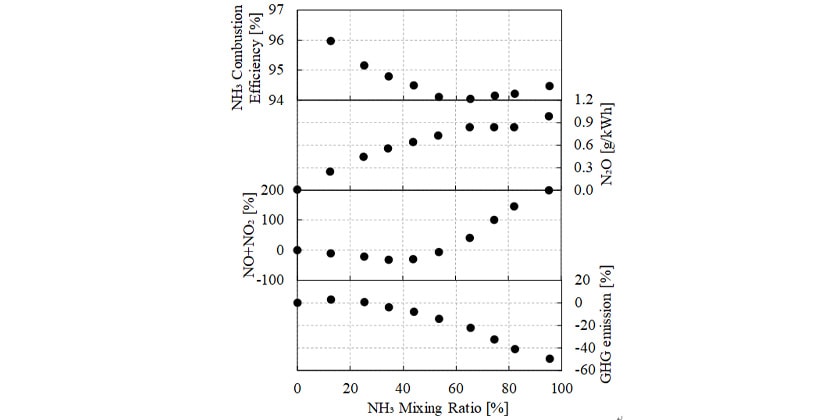
Fig. 3 shows how the NH3 combustion efficiency initially decreased with the increasing mixing ratio but began to rise again once the mixing ratio exceeded 65 %. It is believed that the following two factors influenced this change in NH3 combustion efficiency with mixing ratio: (1) deterioration in ignitability as the mixing ratio increased, and (2) reduction in the amount of unburned NH3 in the exhaust due to high-temperature combustion (as described in section 2.2). In the former case, NH3 combustion efficiency decreased because the amount of diesel as an ignitor decreases as the mixing ratio increases. In the latter case, because the experiment varied the mixing ratio while keeping the intake pressure constant, it is believed that increasing the mixing ratio lowered the air-fuel ratio of the ammonia premixed gas and caused the combustion temperature to rise. This latter factor is believed to explain why NH3 combustion efficiency began to improve again at high mixing ratios.
Emissions of NO+NO2 initially decreased as the mixing ratio increased. This is believed to be due to a reduction in the amount of thermal NO formed in localized regions of high temperature in the diesel diffusion flame. At mixing ratios above 40 %, however, emissions started to increase again, reaching about double the level when running on diesel only. This is believed to be due both to an increase in fuel NOX, and an increase in thermal NO due to the higher adiabatic flame temperature resulting from the lower air-fuel ratio of the ammonia gas component of the fuel.
Emissions of N2O increased as the mixing ratio increased. GHG emissions increased up to a mixing ratio of 30 % before falling again. This is because N2O has such a high GWP that, at low mixing ratios, the global warming impact of N2O outweighs the benefits of using less hydrocarbon fuel.
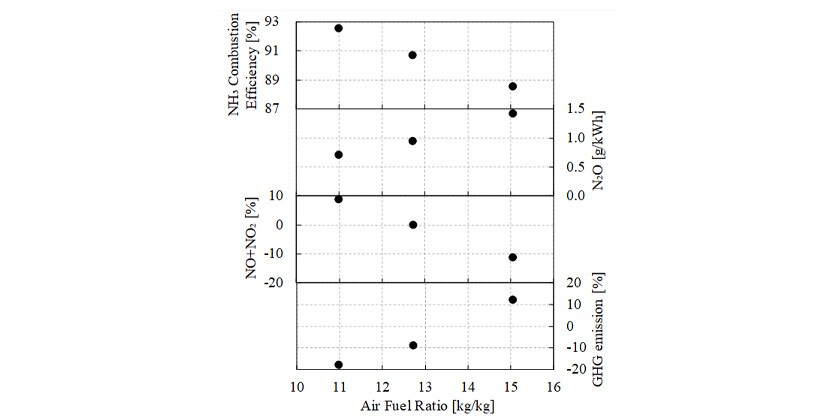
3.2.2.Effects of Air-Fuel Ratio
Next, the experiment looked at the effects on exhaust composition of varying the air-fuel ratio while keeping the ammonia mixing ratio constant at 57 %. Fig. 4 shows the results. As the air-fuel ratio decreased, NH3 combustion efficiency increased and N2O decreased. As indicated by the φ-T map in section 2.2, this is believed to be because a higher equivalence ratio (lower air-fuel ratio) results in a higher combustion temperature, resulting in reduced emissions of the unburned NH3 and N2O produced at lower temperatures. Emissions of NO+NO2 increased as the air-fuel ratio decreased. This is believed to be due to an increase in the thermal NO produced by high-temperature combustion. These results indicate that the effects of air-fuel ratio on exhaust composition observed in this experiment agree with the simulation results presented in section 2.2.
GHG emissions is indicated in the form of relative values compared to running on diesel only shown in Fig. 3. At a mixing ratio of 57 %, GHG emissions are higher than running on diesel only despite the lower CO2 emissions that result from ammonia providing half of the calorific value. This is due to the high level of N2O emissions associated with running a high air-fuel ratio. With a low air-fuel ratio, on the other hand, GHG emissions tend to be lower because less N2O is emitted.
4.Conclusions
Along with determining the basic combustion characteristics of ammonia, this article has demonstrated how the use of ammonia fuel has the potential to reduce GHGs, describing how GHG emissions can be reduced by 49 % using an ammonia-diesel fuel mixture with a mixing ratio of 95 % and an air-fuel ratio of around 6.9. Yanmar plans to conduct further research on GHG reduction measures based not only on combustion, but also in combination of the aftertreatment systems, with the aim of reducing exhaust contaminants.
References
- (1)H. Nakamura, S. Hasegawa, & T. Tezuka, Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile, Combustion and Flame, vol. 185, pp. 16–27 (2017)
- (2)K. Akihama, Y. Takatori, K. Inagaki, S. Sasaki, et al., Mechanism of the Smokeless Rich Diesel Combustion by Reducing Temperature, SAE paper, 2001-01-0655 (2001)
- (3)H. Kobayashi, A. Hayakawa, K.D.K.A. Somarathne, & E.C. Okafor, Science and technology of ammonia combustion, Proceedings of the Combustion Institute, Vol. 37, Issue 1, pp. 109–133 (2019)
-IMPORTANT-
The original technical report is written in Japanese.
This document was translated by Innovation & Technology Division, Technology Strategy Division.
Author
